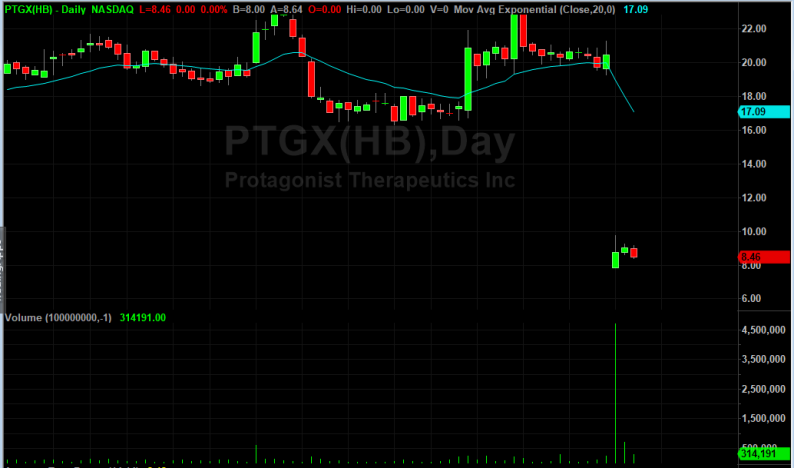
Shares of Protagonist Therapeutics (PTGX) dropped 54% in after-hours trading on Tuesday after announcing it would discontinue phase 2b Propel trial. The pharmaceutical company was conducting a research on a compound called PTG-100 to see if it was a potential treatment for ulcerative colitis.

The company’s decision to bring the study to an early halt came after a scheduled interim analysis indicated that the drug was not functioning, and that proceeding with the study would be in vain.
Although there are several other compounds that the firm is investigating, PTG-100 was its lead compound. Following the news, shares of the pharmaceutical company took a dip tumble losing more than 54% of their value.
Protagonist Therapeutics CEO Comments
Protagonist Therapeutics’ Chief Executive Officer, Dinesh V. Patel said that they were disappointed with the futility-based outcome. Patel said the study was accompanied by an unexpectedly high placebo rate. He however, stated that they would conduct an extensive review of the complete dataset on the totality of patients enrolled in the trial before making any further decisions about the future of PTG-100. The CEO also thanked the patients and investigators who took part in the Propel trial.











Leave A Comment