After opening the day, flat share markets in India witnessed volatile trading activity throughout the day and ended the day firmly in the green. Sectoral indices too ended the day in the green, with stocks in the pharma sector and stocks in the auto sector leading the gains.
At the closing bell, the BSE Sensex stood higher by 287 points (up 0.9%) and the NSE Nifty closed up by 98 points (up 1%). The BSE Mid Cap index ended the day up 1.4%, while the BSE Small Cap index ended the day up by 2.4%.
Asian stock markets finished mixed. As of the most recent closing prices, the Hang Seng was up by 0.2% and the Shanghai Composite was down by 0.2%. The Nikkei 225 was down by 0.3%. Meanwhile, European markets were trading on a positive note. The FTSE 100 was up by 0.2%, The DAX, was up by 1.3% while the CAC 40 was up by 0.7%.
The rupee was trading at Rs 65.01 against the US$ in the afternoon session. Oil prices were trading at US$ 65.17 at the time of writing.
Dr Reddy’s share price ended the day higher, after the company and its American subsidiary, Promius Pharma, LLC announced the filing of a New Drug Application (NDA) for its migraine candidate DFN-02 with the United States Food & Drug Administration (USFDA).
According to the company, the drug, DFN-02 has demonstrated that it can effectively treat pain and associated symptoms during a migraine attack and reduce attack-related functional disability.
Upon approval, the product will be commercialised by Promius Pharma.
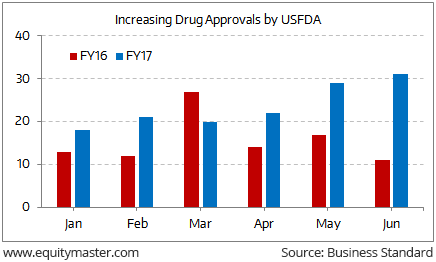
Expediting Drug Approval Process to be a Positive for IndustryIndian pharma companies catering to the US markets are breathing a sigh of relief. After being adversely affected by import bans and the suspension of new drug approvals from manufacturing facilities in the past three years, there has been a sharp pick-up in new drug approvals in FY17.

However, note that USFDA alerts on Indian pharma companies have increased over the past few years. Regulators used to visit the plants every two years. Now they come every eight months. Increasing inspections have led to a total of 41 import alerts in the past eight years – 33 of them (80%) in just the last four years (2013-16). This clearly signifies increased USFDA scrutiny on Indian pharma firms. If that wasn’t enough, increasing pricing pressure in the generics segment has dented realisations.











Leave A Comment