Acceleron Pharma Inc. (XLRN), a clinical stage biopharmaceutical company yesterday announced results from their DART Phase 2 Study of Dalantercept. Dalantercept is used to treat advanced renal cell carcinoma.
The study did not reach its primary endpoints showing a lack of statistical significant increase over the placebo. Acceleron announced that they plan to discontinue the development of dalantercept.
Acceleron Pharma Inc. CEO’s Comments
“We designed a robust Phase 2 study to evaluate the efficacy of dalantercept in combination with anti-VEGF therapy in advanced renal cell carcinoma patients whose disease has progressed on prior anti-VEGF therapy. We are disappointed by the results given the need for new agents that improve outcomes for patients with advanced RCC. We would like to thank the patients, caregivers, investigators, and our team who made the DART study possible,” said Habib Dable, President and Chief Executive Officer of Acceleron. “Based on the lack of efficacy, we are discontinuing the development of dalantercept. We remain focused on the development of luspatercept across multiple Phase 3 and Phase 2 studies and ACE-083 across two neuromuscular diseases, and will continue to pursue additional candidates in areas of high unmet medical need.” Business Wire
XLRN Technical Analysis
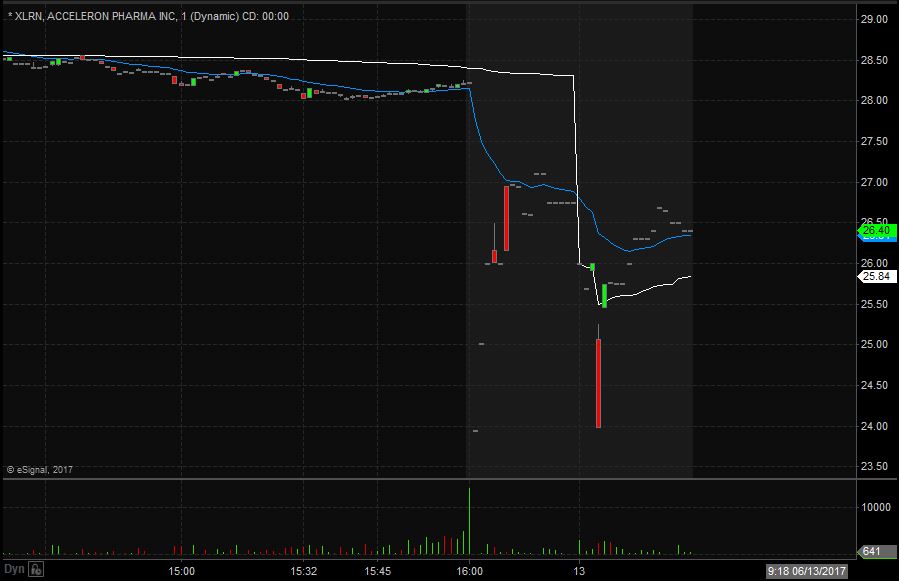
XLRN opened trading yesterday at $28.91 which was down from the previous day’s trading close of $29.04. XLRN closed trading yesterday at $28.23 and spiked down after market to $26.75, equivalent to a 5% decrease from the closing price. Taking a look at the daily chart we can see the last time XLRN traded below these levels we have to go back to May 31st when it traded at $25.51.
Taking a closer look at the daily chart we can see that before the spike down XLRN had already been in an overall downward trend dating back to May 1st when it traded at $33.49. XLRN has a float of 32.76 million shares and traded below the normal daily trading volume on Monday.












Leave A Comment