Anthera Pharmaceuticals, Inc. (ANTH – Free Report) announced positive top-line data from the extension of a phase II BRIGHT-SC study, evaluating key pipeline candidate, blisibimod for treatment of patients with IgA nephropathy (IgAN).
Notably, the BRIGHT-SC study is randomized, double-blind and placebo-controlled, initiated in June 2013, to evaluate the efficacy, safety, tolerability and immunogenicity of blisibimod in patients with IgAN.

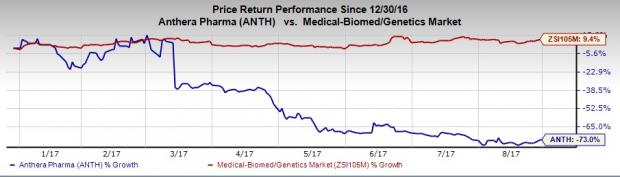
Anthera’s share price movement shows that the stock has significantly underperformed the industry so far this year. The stock has plunged 73% versus the industry’s 9.4% increase during the period.
The extension of phase II BRIGHT-SC study demonstrated a tendency toward lower proteinuria in blisibimod when compared to placebo-treated patients. The study also showed a trend toward preservation of renal function (eGFR) in patients treated with blisibimod. The candidate was well-tolerated during the study compared with placebo.
The trial was conducted on total 58 patients. Patients enrolled in the study were treated for up to 2 years and had the opportunity to complete at least 60 weeks of treatment. Anthera is planning to share these results with the FDA and proceed with a phase III trial for patients with the same indication.
We remind investors that the company has a mid-stage development candidate, Sollpura, acquired from Eli Lilly and Company (LLY – Free Report) in July 2014. The candidate is being developed for treating patients with low digestive enzyme levels or exocrine pancreatic insufficiency (EPI) due to cystic fibrosis or other potential diseases.
The company initiated a phase III SOLUTION study on Sollpura in the third quarter of 2015. In December 2016, the company announced that the study had narrowly missed the primary endpoint of a change in the Coefficient of Fat Absorption (CFA) non-inferiority margin. However, Sollpura achieved a statistical criterion for nitrogen absorption and later on, Anthera too reported positive data from the extension phase of the SOLUTION study earlier in March.













Leave A Comment