On June 12th, Actinium Pharmaceuticals (ATNM) hosted a webinar that provided an update on the pivotal Phase 3 SIERRA trial. In SIERRA, Actinium is investigating Iomab-B as an induction and conditioning agent designed to enable elderly patients with relapsed or refractory acute myeloid leukemia (AML) to receive a bone marrow transplant (BMT). Iomab-B is the only induction and conditioning agent under clinical investigation. This is a truly differentiated approach that has excited some of the top BMT surgeons in the country.
The Case For Iomab-B
Dr. Rajneesh Nath is the Director of Bone Marrow Transplant and Acute Leukemia at Banner Health MD Anderson. Dr. Nath is a principal investigator in the SIERRA trial and participated in Actinium’s webinar update. He provided an excellent background for investors on the challenges of treating elderly patients with AML. This is, unfortunately, a large segment of the affected population.
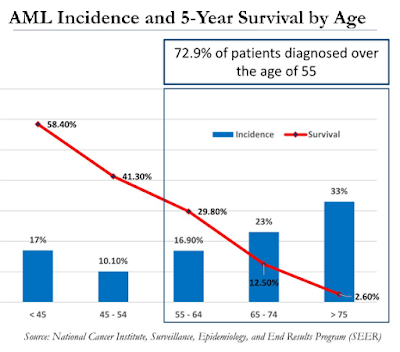
For example, the majority of patients diagnosed with AML are over the age of 55. In fact, the median age is 68 and that creates significant problems in treating these patients due to co-morbid conditions and interactions of concomitant or unrelated medications. As such, the 5-Year survival rate is abysmally low for this older population (see below). New treatment options that are safe and effective are desperately needed.
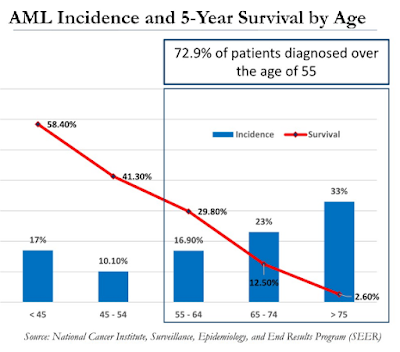
A bone marrow transplant (BMT) is the only curative option for patients with AML. However, in order to qualify for a BMT, the patient must be in complete remission (CR). Transplant surgeons are not going to perform an allogeneic bone marrow transplant on a patient with active disease. As such, not every AML patient is going to qualify for a BMT because complete remission rates for newly diagnosed AML patients are below 50%.
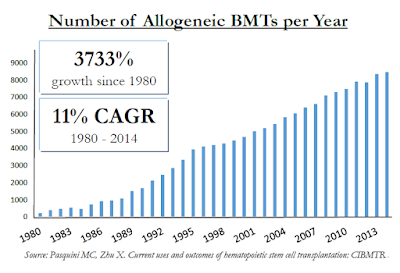
For example, in Celator’s landmark Phase 3 trial investigating Vyxeos™, the CR+CRi rate was 47.7% for Vyxeos vs. only 33.3% for standard of care “7+3” chemotherapy. The majority of patients are not moving onto a BMT despite the fact that it can cure the disease. In fact, there are only roughly 9,000 allogeneic BMT’s in the U.S. each year vs. roughly 21,000 new diagnosed cases of AML. And allogeneic BMT’s are performed on a host of other patients, includes those with multiple myeloma (MM), myelodysplastic syndrome (MDS), non-Hodgkin’s lymphoma (NHL), and acute lymphoblastic leukemia (ALL). These populations sum to over 150,000 individuals in the U.S. Unfortunately, despite allogeneic BMT’s being one of the fastest growing hospital procedures, it falls far short of meeting the medical need.
An important differentiator of Iomab-B is that it can be used in patients with active disease. Unlike standard conditioning agents or patients being readied for a BMT, Iomab-B can be used on refractory or relapsed AML patients. These are patients that are over the age of 55 that might not qualify for myeloablative conditioning due to the potential high toxicities and patients that have tried and failed (or relapsed after) reduced intensity conditioning (RIT) because of the low response rate.









Leave A Comment