TM editors’ note: This article discusses a penny stock and/or microcap. Such stocks are easily manipulated; do your own careful due diligence.
Someone dies of a stroke every 4 minutes in the United States. Globally, 15 million people suffer a stroke every year. It’s debilitating physically and financially—but one little-known company has developed a medical device that hopes to challenge that deadly statistic.
It’s been quietly developing this new technology for 10 years. Now it’s released updated preliminary clinical trial results, and signed a manufacturing deal which could make a significant impact in the medical device industry.
After a decade of painstaking development, we’re now nearing the end of the long road to validation, and for investors who understand this industry, this could be the critical juncture.
The little-known company is CVR Medical (TSX:CVM.V; OTC:CRRVF), and its potentially life-saving device is the Carotid Stenotic Scan (CSS)–a technology designed to detect stenosis within arteries, or Ischemia, which is the leading indicator of strokes.
Of the 15 million people who suffer stroke every year, some 6 million are killed, while 5 million are rendered permanently disabled, according to the World Heart Foundation.
But there has been no cost-effective way to screen for Ischemia.
So, when a biotech company offers a potential solution to help prevent the second-leading cause of death in the world–and then releases positive preliminary clinical trial results, investors listen.
Now it’s hoping to charge out of the gate and take the market by storm once it manages to gain FDA market clearance.
In the meantime, the catalysts are really lining up:
On 7 September, CVR released updated results from its preliminary clinical trial that showed forward progress for the medical device, which we’ve been watching closely for some time. You can view the results from Thomas Jefferson University HERE.
And a few weeks prior, they announced another landmark achievement when they signed a letter of intent to manufacture the CSS. The deal gives CVR state-of-the-art manufacturing capabilities with Canon Virginia, Inc (CVI). This would give CVR immediate scalability, and also adds to its credibility: It’s not the first manufacturing deal the company has sealed.
Our researchers are keeping a close eye on CVR Medical Corp. because we think this is the turning point. Here’s why:
Here are 5 reasons to keep a close eye on CVR Medical (TSX:CVM.V; OTC:CRRVF) in the coming weeks and months:
#1 Catalysts are Lining Up, and News Flow is Gaining Momentum
First, one way to look at the CSS is to think about what 3D seismic imagery did for super quick discoveries in the oil and gas industry. This is exactly what CVR’s sensory system could do for the medical industry in terms of detecting critical stroke symptoms.
This is how it works:
CVR’s Carotid Stenotic Scan (CSS) is a tool to detect blockage within the Carotid Arteries, potentially offering patients and caregivers a device for early detection in a quick and repeatable manner.
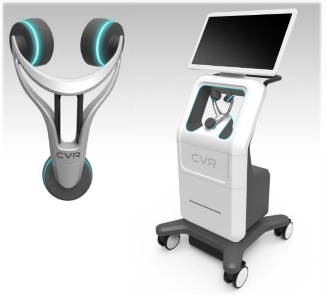
The CSS makes a connection between fluid flow and low frequency sound waves to detect arterial disease or blockage. Blood flowing through the carotid arteries produces wave patterns which are shaped and altered by the presence of irregularities on the inner artery walls.








Leave A Comment