After opening the day higher, stock markets in India have continued their momentum. Sectoral indices are trading on a positive note with stocks in the metal sector and energy sector witnessing maximum buying interest.
The BSE Sensex is trading up 243 points (up 0.8%) and the NSE Nifty is trading up 84 points (up 0.9%). The BSE Mid Cap index is trading up by 1.4%, while the BSE Small Cap index is trading up by 1.5%. The rupee is trading at 63.93 to the US$.
Cadila Healthcare share price is witnessing buying interest today as Zydus Cadila received establishment inspection report from the US health regulator for its manufacturing facility in Ahmedabad.
The development signifies a successful closure of the US Food & Drug Administration (USFDA) audit for the above mentioned facility.
Speaking of USFDA approvals, there’s a sharp pick-up in new drug approvals in 2017. During the period January-July 2017, 129 approvals for generic drugs were made. This is evident from the chart below:
USFDA sweetener for Indian Pharma
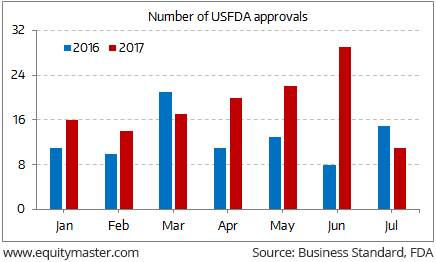
This is 45% higher from 89 approvals made in the corresponding period last year.
As per the Indian Pharmaceutical Alliance, the pace of drug approvals has gained momentum after they complained to FDA about delays last year.
Approvals for drugs have also picked up after USFDA concerns at some of the manufacturing units were addressed. Companies such as Divi’s Laboratories, Cadila Healthcare, Sun Pharmaceuticals and Dr. Reddy’s Laboratories got regulatory clearances for some of their plants during the year. But several companies are still grappling to resolve FDA concerns. Some facilities of Wockhardt, IPCA and Sun Pharma are still under import alert whereas a few units of Divi’s Laboratories and Dr. Reddy’s have received warning letters.
However, FDA’s move to speed up generic drug approvals in a bid to reduce healthcare costs is likely to provide some earnings relief to domestic pharma sector companies.








Leave A Comment